Leading RFID developer creates unique new, high-performance RFID tag for pharmaceutical manufacturers
Checkpoint Systems, a leading global developer of RFID-based technology systems for inventory management applications, has announced a bold new move into the pharmaceutical sector by joining the ambitious DoseID Consortium.
The self-governing, US-based consortium established to unify the industry around an approach to serialized RFID-tagged pharmaceutical products aims to ensure the quality, performance, and interoperability of RFID-tagged products as they move through the supply chain.
The new Spiro Plus M750 label, developed by engineers at Checkpoint and DoseID certified, can be applied to pharmaceutical products at the point of manufacture, enabling them to be tracked throughout the supply chain. The RFID-enabled inventory management solution helps hospitals modernize restocking processes and automate them to save redundant drug spend and ensure patient safety. This will effectively enable hospital pharmacies to better manage their inventory, as well as provide compounders and pharmaceutical manufacturers with accurate data on ‘beyond use’ expiration, medication compounding history, and recalls.
The Spiro Plus M750
Checkpoint Systems, a leading global developer of RFID-based technology systems for inventory management applications, has announced a bold new move into the pharmaceutical sector by joining the ambitious DoseID Consortium.
The self-governing, US-based consortium established to unify the industry around an approach to serialized RFID-tagged pharmaceutical products aims to ensure the quality, performance, and interoperability of RFID-tagged products as they move through the supply chain.
The new Spiro Plus M750 label, developed by engineers at Checkpoint and DoseID certified, can be applied to pharmaceutical products at the point of manufacture, enabling them to be tracked throughout the supply chain. The RFID-enabled inventory management solution helps hospitals modernize restocking processes and automate them to save redundant drug spend and ensure patient safety. This will effectively enable hospital pharmacies to better manage their inventory, as well as provide compounders and pharmaceutical manufacturers with accurate data on ‘beyond use’ expiration, medication compounding history, and recalls.
The new Spiro Plus M750 has been developed exclusively for the challenging environments in which RFID-tagged products will be scanned. It delivers excellent performance in a range of scenarios, including in the presence of RF-inhibiting factors like liquids, metals, and high tag density.
Utilizing the Impinj Monza M750 IC, the label has also achieved ARC’s Spec S Certification, further demonstrating its excellent performance and detection rates. This certification means that pharma manufacturers can use this inlay on their DoseID Certified drugs. A mere 26 x 14mm in size can be applied to a wide variety of products.
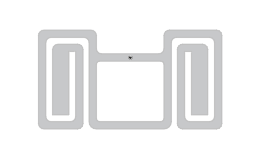
The Spiro Plus M750 design
50+ years of experience
Despite being a relative newcomer to the pharmaceutical sector, Checkpoint has built a wealth of RF and RFID-based experience in numerous other industries, particularly retail where it has supported businesses for over 50 years.
As part of CCL Industries – the world’s largest converter of pressure sensitive and specialty extruded film materials for a wide range of applications used by government institutions and large global customers in the consumer packaging, healthcare & chemicals, consumer electronic device, and automotive markets – Checkpoint has become one the leading global manufacturers of RFID inlays, as well as a developer of high-performance software and hardware.
Checkpoint and CCL Healthcare
Craig Weakley, Director of Applications Development at Checkpoint Systems, said:
“The environments in the pharmaceutical sector make it extremely challenging for RFID to perform well. There is no margin of error and the inlay needs to be developed specifically for the application – there really is no off-the-shelf solution. We are delighted with the accuracy and performance of the Spiro Plus label, which combines the collective and unique experience of our teams at Checkpoint and CCL Healthcare.
“Quality and standards have always been pivotal to the pharmaceutical sector, but the whole industry has been put in the spotlight over the last year due to the pandemic. Solutions that help it quickly and accurately identify fluid-filled containers in densely-packed environments will be hugely important with increased demands on medicines over the coming years.”
Tim Kress-Spatz, Co-Founder of Kit Check, Board Member of DoseID, added:
“We’re thrilled to see Checkpoint join the consortium’s efforts to advance standards around tagged unit-of-use medications and to push the envelope on inlays tailored for the unique and challenging use cases that hospitals face today.”
Checkpoint and CCL Healthcare combine excellence in inlay manufacturing, pharmaceutical label converting, and RFID encoding. This vertical integration of CCL Industries’ divisions helps pave the way for the future of pharmaceuticals and Healthcare RFID tagged products. The integrations will allow CCL Industries to develop unique new solutions for DoseID and other healthcare applications that meet its robust certification criteria.
Newsletter Sign Up
Stay informed on current trends, products, and innovations
Checkpoint Adds its 50 years of Experience to DoseID
- Recent Posts
- All Posts
Printed Literature – Topserts
Pre-Serialized Topserts Folded Leaflets Printed Literature – Topserts A printed...
Read MoreClear Self Adhesive Labels
We offer single-ply, self-adhesive labels made from a variety of...
Read MoreThe Crucial Role of Printed Brand Security Features in Medication
Safeguard pharmaceutical integrity. Learn how robust brand protection thwarts counterfeiting,...
Read MoreRead from our best archive of blogs and solutions.
Image is placeholder
Add button takes you to bog page