Pharmaceutical Label Products
Overview

Pharmaceutical Label Products Overview
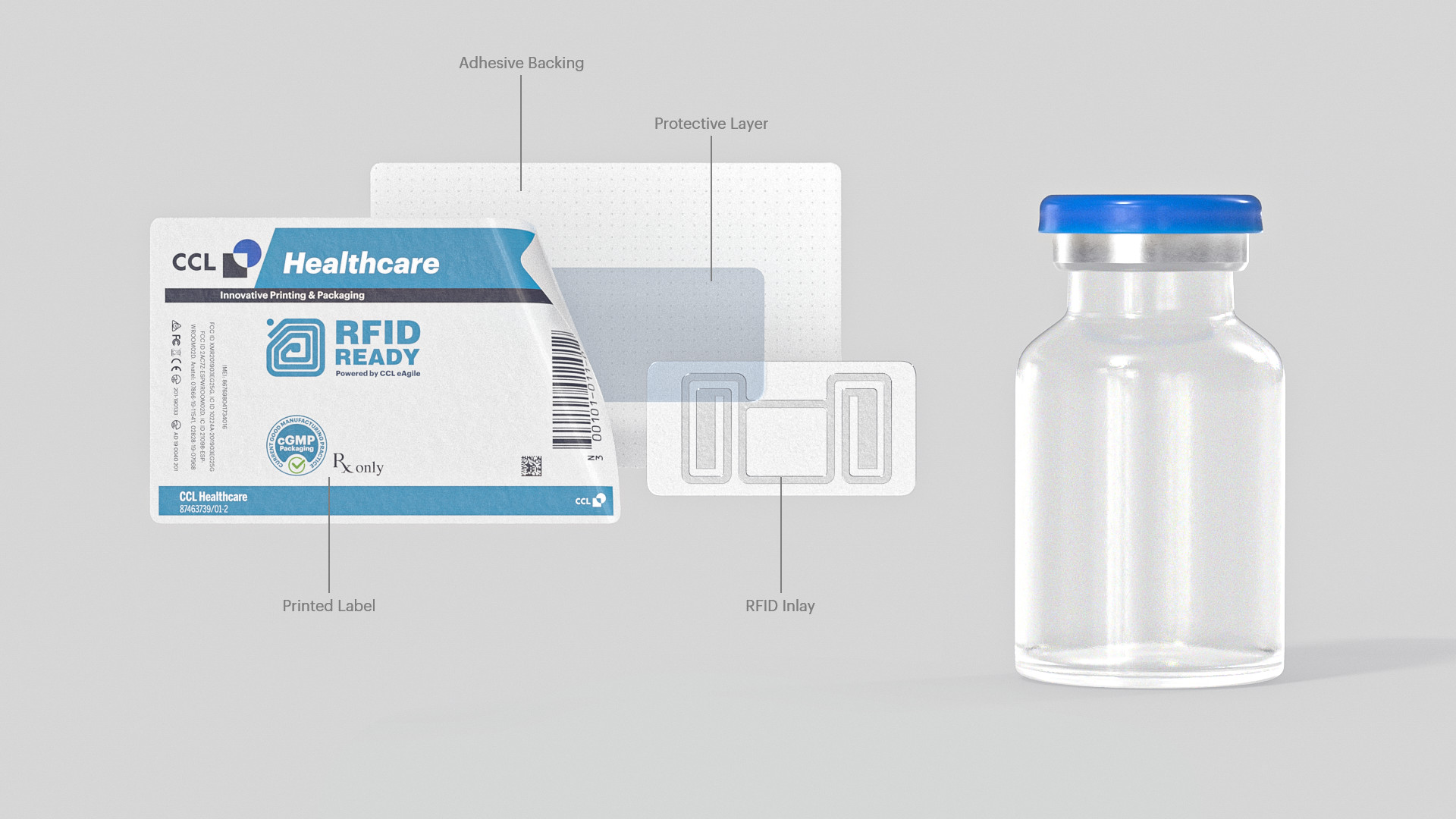
Pharmaceutical label products are customizable and be made with many different features. Custom configurations, designs, and sizes are available. Promotional and functional features can be built into our label products making them an effective marketing tool. High-quality materials, thin foil lamination, specialized adhesives and selected printing inks offer a high degree of flexibility and appeal with our labels. Our label products are printed on pharmaceutical grade paper using high-grade adhesives and are available in black & white, spot color, full color, and the industry’s largest variety of finishings. All our day labels are manufactured in cGMP facilities to ISO Standards with 100% electronic verification.
Why CCL for Pharmaceutical Label Products?
Our pharmaceutical and healthcare labels are printed with the most advanced equipment available in the industry. Pairing our highly trained and qualified staff with our robust SOP’s and cGMP facilities allows us to exceed our customers quality expectations. Our investment in equipment and people have allowed us to continually be the market innovators and grow our pharmaceutical labels and packaging, as well as secondary packaging offerings to meet the unique challenges that the pharmaceutical and healthcare industry demands.
When it comes to quality, CCL Healthcare is the market leader investing heavily into vision verification systems and establishing the standard for pharmaceutical printing in the industry. The standard includes ensuring every product is 100% verified and documented for traceability. We manufacture under cGMP and ISO standards in temperature and humidity control facilities. Our dedicated quality assurance team and production departments follow strict SOPs and CSPs in fully segregated manufacturing areas. Our global presence and manufacturing footprint allows you to go global with 34+ specialized healthcare sites. Our experienced staff provides the highest level of service, technical expertise, and product innovation anywhere you are.
Contact Us
Contact a Packaging Specialist.
Request a Quote
Do you need a quote for Specialty Labels?
News Letter
Stay up-to-date with CCL Healthcare.
- All
- Blog
- Packaging University 101