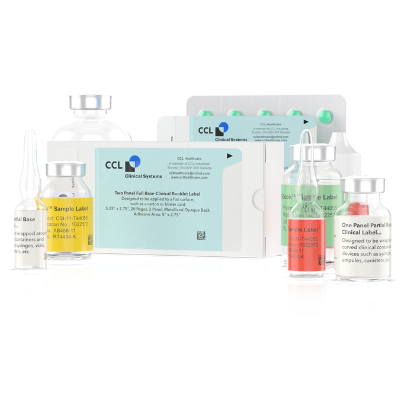
Clinical Trial Labels Overview

Clinical trial labels must maintain accuracy and transparency to enhance patient safety and adhere to regulatory compliance. CCL Clinical, a division of CCL Healthcare, provides high-quality, user-friendly clinical label materials that offer flexible formats, multi-language support and scalability with your clinical trials.
Our Clinical Trial Labels
CCL Clinical offers complete clinical labeling solutions internationally, including clinical booklet labels, conventional clinical labels and unblinded labels. We’re one of the leading clinical trial packaging companies with high-quality clinical trial packaging products, delivering with urgency and accuracy 100% of the time.
Our labels include:
- Booklet labels, which are ideal for international clinical trials that need materials in multiple languages.
- Conventional clinical labels for single or multi-language studies. These labels are fan-folded, blinded and consist of single and multiple panels.
- e-Clinical labels equipped with RFID technology for accessing data on a smart device.
Which Products Right Are for You?
Unsure what you need for your application? Reach out to one of our experts, who will guide you in choosing the best option.
Clinical Label Material Assurance
We provide high-quality clinical trial labels for various trial applications. Our products are specially designed for clinical supply chain divisions, and we carry a large amount of stock of standard items for faster delivery.
If you’re in the market for custom-made labels, our team will design a prototype that includes suitable adhesive combinations and face sheets to meet your specific needs.
We can deliver labels in rolls for hand or automatic application or fanfolds for hand application. When you partner with us, we ensure our clinical trial packaging services are of the highest quality.
What Are the Labeling Requirements for Clinical Trials?
The label must contain specific information to identify the trial and provide clear instructions for use. Here are the key components typically required on the label:
The label should prominently display the name and address of the organization or entity sponsoring the clinical trial. This information helps identify who is conducting and overseeing the study.
Each clinical trial gets a unique protocol number, which serves as an identifier for the study. Including the protocol number on the label helps professionals reference and follow the correct study.
The clinical trial phase is an important piece of information indicating the stage of development and testing the investigational product has reached. The label should clearly state the phase — phase I, phase II or phase III — to provide context for the study.
The label on the clinical packaging must provide clear and concise instructions for how to use and administer the product. This includes information on dosage, frequency, route of administration and any special considerations or precautions.
Pressure Sensitive Questions?
Not sure of what you need for your application ? One of our packaging specialist will be glad to help.
Why Partner With CCL Clinical?
CCL Clinical provides innovative clinical trial packaging solutions that improve data management and the patient experience. For almost seven decades, CCL has been trusted to manufacture and distribute pharmaceutical packaging to hospitals and medical facilities. With our sustainability initiatives and trusted expertise, we’re at the forefront of providing cost-effective, durable solutions.
Trusted Services
We have a strict quality policy, are ISO 9001 Certified and adhere to cGMP guidelines. You can be confident when you do business with us.
Global Presence
CCL has manufacturing sites worldwide, allowing us to serve a global audience and ship anywhere in the world, offering excellent customer service throughout the process.
Supply Chain Management
CCL understands efficient supply chain practices and can order your products to your demands. We keep your facility productive with fast, quality services.
Wide Inventory
We have a wide selection of clinical label materials and various other pharmaceutical packaging solutions.
Custom Options
You can tailor our clinical trial labels to your specific requirements, allowing you to differentiate and protect your brand.
Responsive Service
Our team is ready to assist and always provides individualized, responsive service.
Request Your Quote Today
CCL will work with you, from design to commercial launch. We’re a complete solution provider that assists you in staying compliant while prioritizing patient needs.
To discuss your packaging requirements or request a quote, fill out this online form today.