Human Factors Role in
Designing Instructions for Use
Human factors and instructions for use (IFUs) play a critical role in ensuring the safe and effective use of pharmaceutical packaging. In today’s highly regulated pharmaceutical industry, the design of packaging and IFUs must be carefully considered to ensure that patients can use the medication safely and correctly.
Human factors are the study of how people interact with products, systems, and environments. This field has become increasingly important in pharmaceutical packaging design as the complexity of medication regimens has increased. The packaging design must be user-friendly, considering the various needs and abilities of the end-user, such as elderly or visually impaired patients.
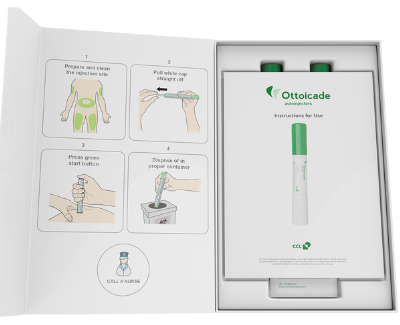
IFUs for Packaging Design
IFUs are an essential aspect of packaging design, providing instructions to the user on how to use the medication safely and effectively. IFUs must be clear, concise, and easy to understand, regardless of the user’s educational background or language proficiency. To ensure that packaging and IFUs meet the needs of patients, pharmaceutical manufacturers conduct usability testing during the design phase. Usability testing involves observing users as they interact with the packaging and IFUs to identify any design flaws and areas for improvement. Testing is typically conducted with a range of users, including those with different abilities and cultural backgrounds, to ensure that the packaging and IFUs are accessible to all users.
Human factors and IFUs are also critical in minimizing medication errors, which can have serious consequences. According to the World Health Organization, medication errors affect approximately one in ten patients worldwide, resulting in prolonged hospital stays, disability, and even death. By considering human factors and designing user-friendly packaging and IFUs, manufacturers can reduce the likelihood of medication errors and improve patient safety.
In conclusion, human factors and instructions for use are essential considerations in the design of pharmaceutical packaging. Designing packaging that is user-friendly and providing clear, concise instructions for use can improve patient safety and reduce the risk of medication errors. Manufacturers must conduct usability testing to ensure that packaging and IFUs are accessible to all users, regardless of their abilities or cultural backgrounds. By prioritizing human factors and IFUs, pharmaceutical manufacturers can ensure that patients can use their medication safely and effectively.