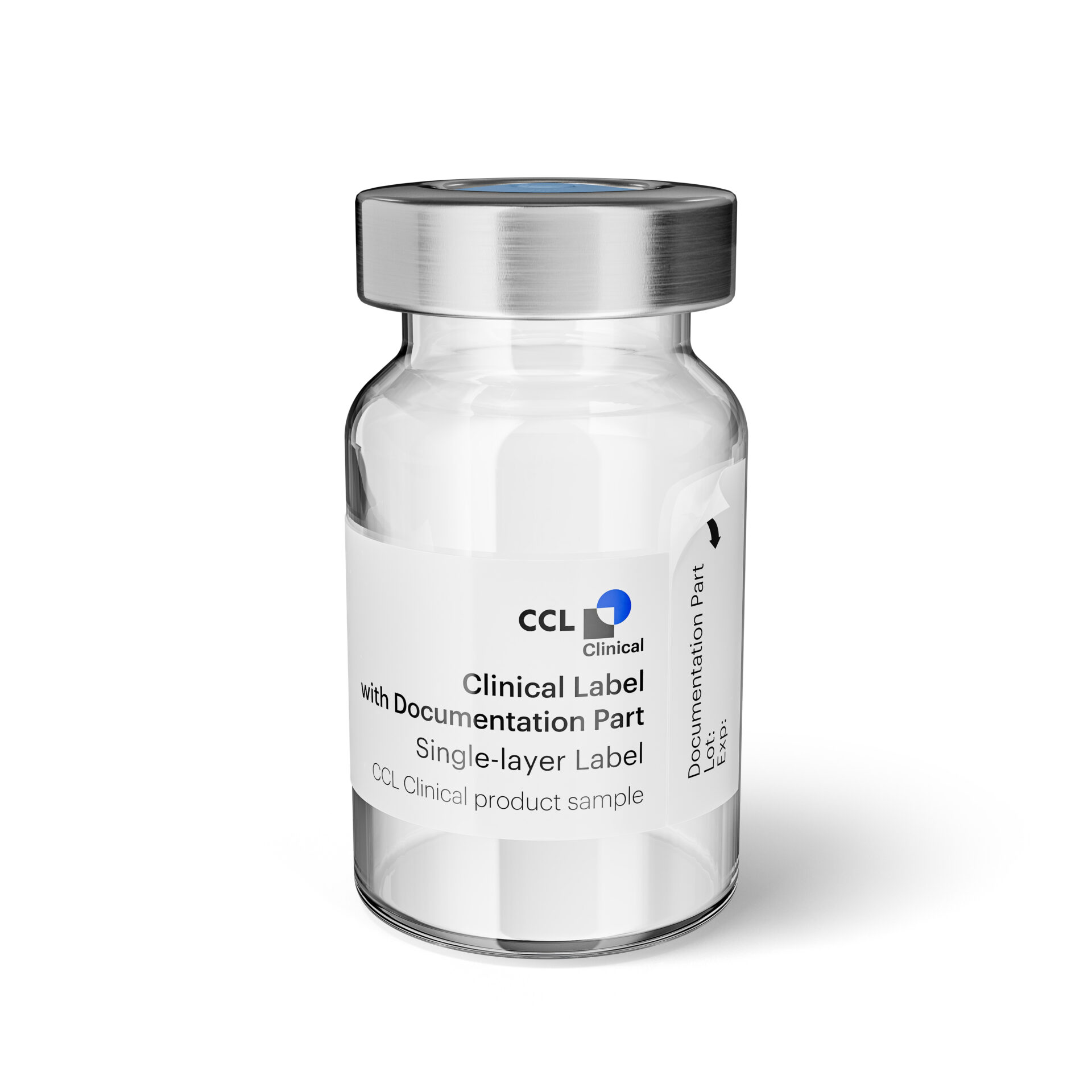
Clinical Labels
With Documentation Parts

Tailored for various markets and regulatory requirements
Our reliable Clinical Labels with Documentation Parts ensure your trial meets all necessary standards. These labels feature self-adhesive, writable documentation parts directly on the IMP, making essential information readily available for participants records. By providing clear, accessible documentation, our labels enhance compliance and streamline trial processes.
Our Clinical Labels with Documentation Parts are available in various formats, including Booklet Labels, Multi-Layer Labels, and Single-Layer Labels, to suit your specific needs.

Clinical Label with Documentation Part
Key Features
Applications
Our Clinical Labels with Documentation Parts are ideal for a variety of container types including:
- Bottles
- Vials
- Ampoules
- Pens
- Syringes
- Folding Cartons
- Canisters
- Inhalers
- Tubes
- Blister Cards
Why Choose Us
With a deep understanding of the clinical trial industry and its unique requirements, CCL Clinical is committed to providing labeling solutions that meet the highest standards of quality, compliance, and usability. Our Clinical Labels with Documentation Parts clearly display critical data directly on the IMP to facilitate easy access and reference.
Enhance the reliability, efficiency, and safety of your clinical trials with our Clinical Labels with Documentation Parts. Contact us today to discuss your specific needs and discover how our innovative labeling solutions can support the success of your clinical research.