Clinical Booklet Labels
Overview

For Labeling IMPs in Round and Square Containers
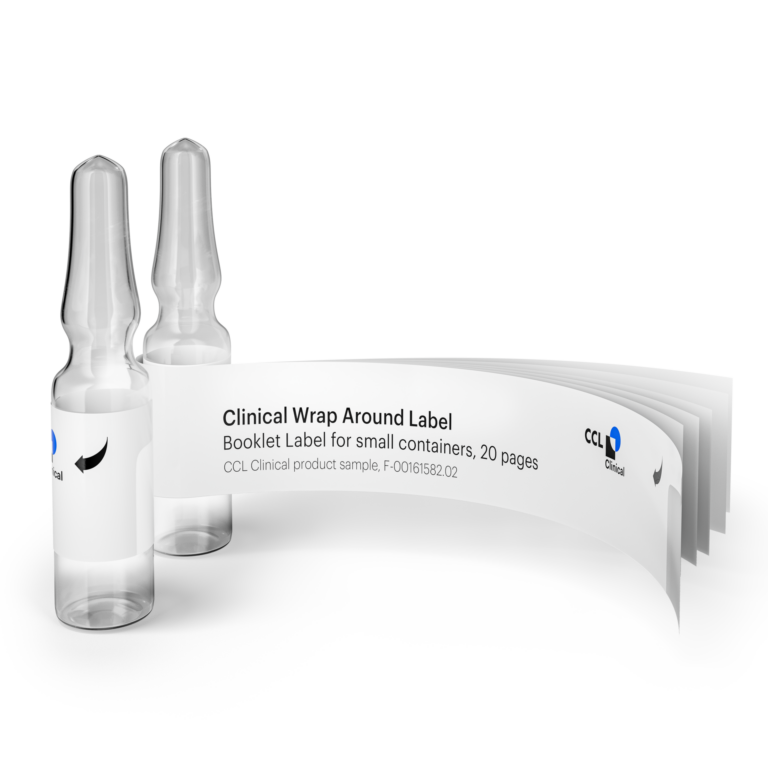
Streamline your clinical trial processes with our innovative Clinical Booklet Labels, specifically designed to meet the complex needs of global clinical research. These Booklet Labels provide a practical and efficient solution for conveying extensive information in a reader-friendly format, with up to 113 pages on a compact surface, ensuring compliance and enhancing the organization of your clinical trials.
Clinical Booklet Labels can be adhered to a variety of packages used in multinational trials. The partial or split base design of these labels allows them to be affixed to round, and cylindrical containers such as bottles, vials, ampoules, pens, and syringes without the need for folding, thanks to the strategic gap.
Applications
Our Clinical Booklet Labels are ideal for a variety of clinical trial applications, including:
- Bottles
- Vials
- Ampoules
- Pens
- Syringes
- Tubes
Clinical wrap Around Labels: Our Clinical Booklet Labels feature a multi-layered design that allows for extensive information to be included in a small space. This is particularly beneficial for clinical trials where detailed instructions, multiple languages, and regulatory information are required.
Clinical Labels for Cold Chain and Cryogenic Application: Made from robust, high-quality materials, our Clinical Booklet Labels are designed to withstand various environmental conditions, including exposure to moisture, chemicals, and temperature fluctuations. This ensures that all information remains legible and intact throughout the trial duration.
Clinical Variable Data Printing: We offer a wide range of customization options to cater to the specific needs of your clinical trial. Whether you need barcodes, QR codes, or Data Matrix codes, we can tailor our Clinical Booklet Labels to your requirements.
Our Clinical Booklet Labels are designed in accordance with all relevant regulatory standards, including FDA, EMA, and ICH guidelines, and recommended by ISPE. This ensures that your clinical trials can proceed smoothly without any compliance issues related to labeling.
The user-friendly design of our Clinical Booklet Labels allows for easy application and secure attachment to various container types. The labels can be easily peeled back to reveal additional information without compromising the integrity of the label or the container.
With the ability to include multiple pages of information, our Clinical Booklet Labels significantly enhance information management in clinical trials. This includes patient instructions, dosage information, safety warnings, and study protocols, all in one convenient label.
Clinical Booklet Labels can include security features such as holograms or seals to ensure the integrity of the information and prevent tampering or counterfeiting.
Key Features of Our Booklet Labels
Why Choose Us
Drawing on our outstanding knowledge in delivering labeling solutions for the clinical trial industry, CCL Clinical understands the critical importance of clear, compliant, and durable labeling. Our Booklet Labels are designed to support the rigorous demands of clinical research, ensuring that all necessary information is clearly conveyed and easily accessible.
Improve the efficiency, compliance, and overall success of your clinical trials with our advanced Booklet Labels. Contact us today to discuss your specific needs and discover how our tailored labeling solutions can benefit your clinical research.