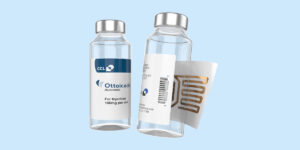
Recap: Single Unit Doses with RFID

Pharmaceutical Manufacturers In Need of Visibility
Pharmaceutical manufacturers are looking for labels that track and trace medication throughout the supply chain all the way until it’s administered to the patient. Furthermore, this can be accomplished through RFID enabled products. These insights bring valuable information used for several functions. Moreover, using Kit Check, hospitals can optimize inventory, gain better visibility into medication usage levels in real-time, and analyze dispense patterns.
What RFID Enabled Products Can Do for You
The CCL Packaging University Course dives into why RFID-enabled drug products and cloud data solutions are a packaging game changer. Generally, for pharmacists, Kit Check allows providers to practice at the top of their license, rather than spend time manually restocking and managing the medication supply chain. For pharmaceutical companies to be competitive, Kit Check labels add value by being able to track the life cycle of the product. Therefore, the RFID labels add features highly valued by hospitals and pharmacies with several safety protocols and compliance standards to meet. For pharmaceutical manufacturers, this is equally important to set pharmaceutical product apart from their competitors. O.R.s and pharmacists are more likely to stock a RFID-enabled drug product versus another pharmaceutical product that does not have the same traceability capabilities. Thus, the decision becomes value-driven, making it a necessity for drug manufacturers to have in order to stay competitive.
RFID Enabled Medications Coupled with Cloud Data Solutions
In the course, we learn that Kit Check’s RFID-enabled drug products and cloud data solutions are bringing unit-dose medication intelligence into the hospital and back into the drug supply chain. These insights are valuable to manufacturers, hospitals, and patients. Therefore, selecting medications labeled with Kit Check capabilities is now the ultimate deciding factor as it is becoming a compliance prerequisite and an inventory control standard. In the presentation, Lori Murphy from Kit Check demonstrates the information collected and maintained in Kit Check’s software. The presentation shows different use cases and products available. There is a thought-provoking question and answer session where the attendees ask the presenter follow-up questions about Kit Check’s cloud technology in conjunction with the CCL RFID solution. Therefore, this course is great for anyone who works in the pharmaceutical manufacturing space.
Newsletter Sign Up
Stay informed on current trends, products, and innovations
- Recent Posts
- All Posts
Basics of Extended Content Labels
ECLs can be life-saving and help your customers gain more...
Read MoreTypes of RFID Tags
Radio-frequency identification (RFID) tags are crucial tools in tracking and...
Read More5 Smart packaging trends to Watch
Technological advancements have fueled the emergence and growth of smart...
Read MoreRead from our best archive of blogs and solutions.
Image is placeholder
Add button takes you to bog page