Folding Carton with Product Display window

Folding cartons with product display windows are particularly effective for products that are visually appealing or have unique features that are not immediately apparent from the packaging alone. The window allows customers to see the product in detail and may encourage them to make a purchase based on the product’s appearance.
Multi-product Pack Folding Carton

Multi-product pack folding cartons can be designed in a variety of shapes and sizes to accommodate different products, from cosmetics to food items. The packaging typically includes dividers or partitions to keep the products separate and prevent damage during transport and handling.
Folding Cartons Vial Packs
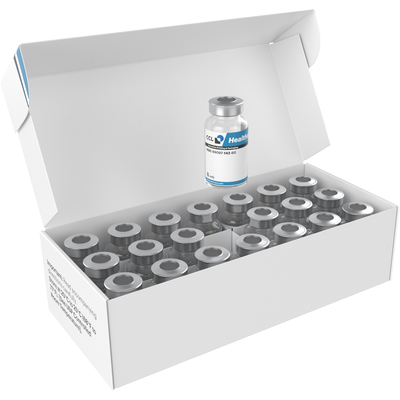
Folding cartons for multiple vials may also include additional features, such as tamper-evident seals, to ensure the integrity of the medication inside. This is particularly important in the pharmaceutical industry, where product safety and quality are of the utmost importance.
Folding Cartons for Syringes
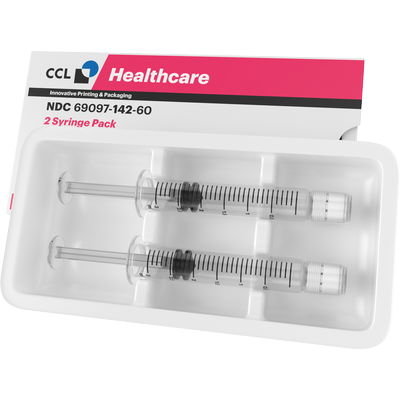
Pharmaceutical syringe cartons are an essential component of the packaging process for pharmaceutical products. They play a crucial role in ensuring the safety and efficacy of injectable medications, as well as providing protection during transport and storage
Human Factors Role in IFU’s

Human factors and instructions for use (IFUs) play a critical role in ensuring the safe and effective use of pharmaceutical packaging. In today’s highly regulated pharmaceutical industry, the design of packaging and IFUs must be carefully considered to ensure that patients can use the medication safely and correctly.
What are Medication Guides

Medication guides, also known as patient information leaflets or package inserts, are essential documents that come with prescription drugs.